INTRODUCTION
A burgeoning need exists today for small, compact, reliable, lightweight and self-contained rugged power supplies to provide electrical power in such applications as electric automobiles, homes, industrial, agricultural, recreational, remote monitoring systems, spacecraft and deep-sea probes. Radar, advanced communication satellites and especially high technology weapon platforms will require much larger power sources than today’s power systems can deliver. For very high-power applications, nuclear reactors appear to be the answer. However, for the intermediate power range, 10 to 100 kilowatts (kW), the nuclear reactor presents formidable technical problems.
Because of the short and unpredictable lifespan of chemical batteries, however, regular replacements would be required to keep these devices humming. Also, enough chemical fuel to provide 100 kW for any significant period of time would be too heavy and bulky for practical use. Fuel cells and solar cells require little maintenance.
Thus the demand to exploit radioactive energy has become inevitably high. Several methods have been developed for the conversion of radioactive energy released during the decay of natural radioactive elements into electrical energy. A grapefruit-sized radioisotope thermo-electric generator that utilized heat produced from alpha particles emitted as plutonium-238 decay was developed during the early 1950s.
Since then nuclear has taken a significant consideration in the energy source of the future. Also, with the advancement of technology the requirement for lasting energy sources has increased to a great extent. The solution to the long-term energy source is, of course, nuclear batteries with a life span measured in decades and the potential to be nearly 200 times more efficient than the currently used ordinary batteries. These incredibly long-lasting batteries are still in the theoretical and developmental stage of existence, but they promise to provide clean, safe, almost endless energy.
Unlike conventional nuclear power generating devices, these power cells do not rely on a nuclear reaction or chemical process and do not produce radioactive waste products. Nuclear battery technology is geared towards applications where power is needed in inaccessible places or under extreme conditions.
The researchers envision its uses in pacemakers and other medical devices that would otherwise require surgery to repair or replace. Additionally, deep-space probes and deep-sea sensors, which are beyond the reach of repair, would benefit from such technology. In the near future, this technology is said to make its way into commonly used day-to-day products like mobile and laptops and even the smallest of devices used at home. Surely these are the batteries of the near future.
HISTORICAL DEVELOPMENTS
The idea of a nuclear battery was introduced at the beginning of 1950 and was patented on March 3rd, 1959 to the tracer lab. Even though the idea was given more than 30 years before, no significant progress was made on the subject because the yield was very less.
A radioisotope electric power system developed by inventor Paul Brown was a scientific breakthrough in nuclear power. Brown’s first prototype power cell produced 100,000 times as much energy per gram of strontium -90(the energy source) than the most powerful thermal battery yet in existence. The magnetic energy emitted by the alpha and beta particles inherent in nuclear material. Alpha and beta particles are produced by the radioactive decay of certain naturally occurring and man-made nuclear materials (radionuclides). The electric charges of the alpha and beta particles have been captured and converted to electricity for existing nuclear batteries, but the amount of power generated from such batteries has been very small.
Alpha and beta particles also possess kinetic energy, by successive collisions of the particles with air molecules or other molecules. The bulk of the R &D of nuclear batteries in the past has been concerned with this heat energy which is readily observable and measurable. The magnetic energy given off by alpha and beta particles is several orders of magnitude greater than the kinetic energy or the direct electric energy produced by these same particles. However, the myriads of tiny magnetic fields existing at any time cannot be individually recognized or measured. This energy is not captured locally in nature to produce heat or mechanical effects, but instead, the energy escapes undetected.
Brown invented an approach to “organize” these magnetic fields so that great amounts of otherwise unobservable energy could be harnessed. The first cell constructed (that melted the wire components) employed the most powerful source known, radium-226, as the energy source.
The main drawback of Mr Brown’s prototype was its low efficiency, and the reason for that was when the radioactive material decays, many of the electrons are lost from the semiconductor material. With the enhancement of more regular pitting and the introduction of better fuels, nuclear batteries are thought to be the next generation batteries and there is hardly any doubt that these batteries will be available in stores within another decade.
BETAVOLTAICS
Betavoltacis is an alternative energy technology that promises vastly extended battery life and power density over current technologies. Betavoltaics are generators of electrical current and affect a form of a battery, which uses energy from a radioactive source emitting beta particles (electrons). The functioning of a betavoltaics device is somewhat similar to a solar panel, which converts photons (light) into electric current.
The betavoltaic technique uses a silicon wafer to capture electrons emitted by a radioactive gas, such as tritium. It is similar to the mechanics of converting sunlight into electricity in a solar panel. The flat silicon wafer is coated with a diode material to create a potential barrier. The radiation absorbed in the vicinity of potential barriers like a p-n junction or a metal-semiconductor contact would generate separate electron-hole pairs which in turn flow in an electric circuit due to the voltaic effect. Of course, this occurs to a varying degree in different materials and geometries.
A pictorial representation of a basic Betavoltaic conversion is shown in the figure. Electrode A (P-region) has a positive potential while electrode B (N-region) is negative with the potential difference provided by me conventional means.
The junction between the two electrodes is comprised of a suitably ionisable medium exposed to decay particles emitted from a radioactive source.
The energy conversion mechanism for this arrangement involves energy flow in different stages:
Stage 1:- Before the radioactive source is introduced, a difference in potential between two electrodes is provided by conventional means. An electric load RL is connected across electrodes A and B. Although a potential difference exists, no current flows through the load RL because the electrical forces are in equilibrium and no energy comes out of the system. We shall call this ground state E0.
Stage 2:- Next, we introduce the radioactive source, say a beta emitter, to the system. Now, the energy of the beta particle Eb generates electron-hole pair in the junction by imparting kinetic energy which knocks electrons out of the neutral atoms. This amount of energy E1 is known as the ionization potential of the junction.
Stage 3:- Further the beta particle imparts an amount of energy in excess of ionization potential. This additional energy raises the electron energy to an elevated level of E2. Of course, the beta [particle does not impart its energy to a single ion pair, but a single beta particle will generate as many as thousands of electron-hole pairs. The total number of ions per unit volume of the junction is dependent upon the junction material.
Stage 4:- Next, the electric field present in the junction acts on the ions and drives the electrons into electrode A. the electrons collected in electrode A together with the electron deficiency of electrode B establish Fermi voltage between the electrodes. Naturally, the electrons in electrode A seek to give up their energy and go back to their ground state (law of entropy).
Stage 5:- the Fermi voltage derives electrons from electrode A through the load where they give up their energy in accordance with conventional electrical theory. A voltage drop occurs across the load as the electrons give an amount of energy E3. Then the amount of energy available to be removed from the system is
E3= Eb - E1 – L1-L2
Where L1 is the converter loss and L2 is the loss in the electrical circuit.
Stage 6:- the electrons, after passing to the load have an amount of energy E4.from the load, the electrons are then driven into the electrode B where it is allowed to recombine with a junction ion, releasing the recombination energy E4 in the form of heat this completes the circuit and the electron has returned to its original ground state.
The end result is that the radioactive source acts as a constant current generator. Then the energy balance equation can be written as
E0=Eb –E1 –E3-L1-L2
Until now betavoltaics has been unable to match solar-cell efficiency. The reason is simple: when the gas decays, its electrons shoot out in all directions. Many of them are lost. A new Betavoltaic device using porous silicone diodes was proposed to increase their efficiency. The flat silicon surface, where the electrons are captured and converted to a current and turned into a 3- dimensional surface by adding deep pits. Each pit is about 1 micron wide. That is four hundred thousandths of an inch. They are more than 40 microns deep. When the radioactive gas occupies these pits, it creates the maximum opportunity for harnessing the reaction.
DIRECT CHARGING GENERATORS

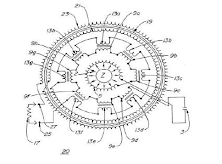
In this type, the primary generator consists of a high –Q LC tank circuit. The energy imparted to radioactive decay products during the spontaneous disintegrations of radioactive material is utilized to sustain and amplify the oscillations in the high-Q LC tank circuit the circuit inductance comprises a coil wound on a core composed of radioactive nuclides connected in series with the primary winding of a power transformer. The core is fabricated from a mixture of three radioactive materials which decay primarily by alpha emission and provides a greater flux of radioactive decay products than the equivalent amount of single radioactive nuclei. The figure is a schematic diagram of an LC-equivalent resonant circuit.
The processes involved in the conversion of the energy released by the spontaneous disintegration of radioactive material into electrical energy are numerous and complex. Materials that are naturally radioactive, decay by the emission of either an alpha particle or a beta particle and gamma rays may accompany either process. Radioactive materials that decay primarily by alpha particle emission are preferred as inductor core 7 materials. Alpha particles are emitted at very high speeds, in the order of 1.6*107 meters per second (m/s) and consequently have very high kinetic energy. Alpha particles emitted in radium, for example, decays are found to consist of two groups, those with a kinetic energy of 48.79*105 electron volts (eV) and those having an energy of 46.95*105 electron volts. This kinetic energy must be dissipated when the alpha particles are absorbed by the conductor forming inductor 5. During the absorption process, each alpha particle will collide with one or more atoms in the conductor knocking electrons from their orbits and imparting some kinetic energy to the electrons. This results in an increased number of conduction electrons in the conductor thereby increasing its conductivity.
Since the alpha particle is a positively charged ion, while the alpha particle is moving it will have an associated magnetic field. When the alpha particle is stopped by the conductor, the magnetic field will collapse thereby inducing a pulse of current in the conductor producing a net increase in the current flowing in circuit 1. Also, there will be additional electrons stripped from orbit due to ionization reduced by the positively charged alpha particles.
The nuclear battery is constructed in a cylindrical configuration. Inductor 5 is constructed of copper wire wound in a single layer around the radioactive core 7. Decay products, such as alpha particles, are emitted radially outward from core 7 as indicated by arrow 2 to be absorbed by the copper conductor forming inductor 5. Eight transformers are arranged in a circular pattern to form a cylinder concentric with and surrounding inductor 5. The transformers have primary windings 9a-9h connected in series which are then connected in series with inductor 5 and capacitor 3 to form an LCR circuit. The central core 7, inductor5 and the eight transformers 15 are positioned within a cylindrical shaped container 19. Copper wire is wound in a single layer on the outside wall and the inside wall of cylinder 19 to form windings 23 and21 respectively. The transformers 15, secondary windings 13a-13h and windings 21 and 23 are connected in series to output terminals 25 and 27. The configuration of inductor 5 is designed to ensure maximum eradication of the copper conductor by the radioactive core source 7. The cylindrical configuration of the power transformer ensures maximum transformer efficiency with minimum magnetic flux leakages.
OPTO ELECTRICS
An optoelectric nuclear battery has been proposed by researchers of the Kurchatov institute in Moscow. A beta emitter such as technetium-99 is strontium-90 is suspended in a gas or liquid containing luminescent gas molecules of the exciter type, constituting “dust plasma”. This permits a nearly lossless emission of beta electrons from the emitting dust particles for excitation of the gases whose exciter line is selected for the conversion of the radioactivity into a surrounding photovoltaic layer such that a comparably lightweight low pressure, high-efficiency battery can be realized. These nuclides are low-cost radioactive nuclear power reactors. The diameter of the dust particles is so small (a few micrometres) that the electrons from the beta decay leave the dust particles nearly without loss. The surrounding weakly ionized plasma consists of gases or gas mixtures (e.g. krypton, argon, xenon) with exciter lines, such that a considerable amount of the energy of the beta electrons is converted into this light the surrounding walls contain photovoltaic layers with wide forbidden zones as an egg. Diamond converts the optical energy generated from the radiation into electric energy.
The battery would consist of an exciter of argon, xenon, or krypton (or a mixture of two or three of them) in a pressure vessel with an internal mirrored surface, finely-ground radioisotope and an intermittent ultrasonic stirrer, illuminating photocell with a band gap tuned for the exciter. When the electrons of the beta active nuclides (e.g. krypton-85 or argon-39) are excited, in the narrow exciter band at a minimum thermal loss, the radiations so obtained is converted into electricity in a high band gap photovoltaic layer (e.g. in a p-n diode) very efficiently the electric power per weight compared with existing radionuclide batteries can then be increased by a factor 10 to 50 and more. If the pressure vessel is carbon fibre/epoxy the weight-to-power ratio is said to be comparable to an air-breathing engine with fuel tanks. The advantage of this design is that precision electrode assemblies are not needed and most beta particles escape the finely-divided bulk material to contribute to the battery's net power. The disadvantage consists in the high price of the radionuclide and in the high pressure of up to 10MPa (100bar) and more for the gas that requires an expensive and heavy container.
The major criterions considered in the selection of fuels are:
* Avoidance of gamma in the decay chain
· * Half-life
· * Particle range
· * Watch out for (alpha, n)reactions
Any radioisotope in the form of a solid that gives off alpha or beta particles can be utilized in the nuclear battery. The first cell constructed (that melted the wire components) employed the most powerful source known, radium-226, as the energy source. However, radium-226 gives rise through decay to the daughter product bismuth-214, which gives off strong gamma radiation that requires shielding for safety. This adds a weight penalty to mobile applications.
 · Strontium-90 gives off no gamma radiation so it does not necessitate the use of thick lead shielding for safety.strontium-90 does not exist in nature, but it is one of the several radioactive waste products resulting from nuclear fission. The utilizable energy from strontium-90 substantially exceeds the energy derived from nuclear fission which gave rise to this isotope.
· Strontium-90 gives off no gamma radiation so it does not necessitate the use of thick lead shielding for safety.strontium-90 does not exist in nature, but it is one of the several radioactive waste products resulting from nuclear fission. The utilizable energy from strontium-90 substantially exceeds the energy derived from nuclear fission which gave rise to this isotope.
ADVANTAGES
The most important feat of nuclear cells is the life span they offer, a minimum of 10 years! This is whopping when considering that it provides non-stop electric energy for the seconds spanning these 10 long years, which may simply mean that we keep our laptops or any handheld devices switched on for 10 years nonstop. Contrary to fears associated with conventional batteries nuclear cells offer reliable electricity, without any drop in the yield or potential during their entire operational period. Thus the longevity and reliability coupled together would suffice the small factored energy needs for at least a couple of decades.
The largest concern about nuclear batteries comes from the fact that it involves the use of radioactive materials. This means throughout the process of making a nuclear battery to final disposal, all radiation protection standards must be met. Balancing the safety measures such as shielding and regulation while still keeping the size and power advantages will determine the economic feasibility of nuclear batteries. Safeties with respect to the containers are also adequately taken care of as the battery cases are hermetically sealed. Thus the risk of safety hazards involving radioactive material stands reduced.
DRAWBACKS
First and foremost, as is the case with most breathtaking technologies, the high initial cost of production involved is a drawback but as the product goes operational and gets into bulk production, the price is sure to drop. The size of nuclear batteries for certain specific applications may cause problems, but can be done away with as time goes by. For example, the size of Xcell used for laptop batteries is much more than the conventional battery used in laptops.
Though radioactive materials sport high efficiency, the conversion methodologies used presently are not much of any wonder and at best match conventional energy sources. However, laboratory results have yielded much higher efficiencies, but are yet to be released into the alpha stage.
A minor blow may come in the way of existing regional and country-specific laws regarding the use and disposal of radioactive materials. As these are not unique worldwide and are subject to political horrors and ideology prevalent in the country. The introduction legally requires these to be scrapped or amended. It can be however hoped that, given the revolutionary importance of this substance, things would come in favour gradually.
Above all, to gain social acceptance, new technology must be beneficial and demonstrate enough trouble-free operation that people begin to see it as a “normal” phenomenon. Nuclear energy began to lose this status following a series of major accidents in its formative years. Acceptance accorded to nuclear power should be trust-based rather than technology-based. In other words, acceptance might be related to public trust of the organizations and individuals utilizing the technology as opposed to based on an understanding of the available evidence regarding the technology.
APPLICATIONS
Space applications
In space applications, nuclear power units offer advantages over solar cells, fuel cells and ordinary batteries because of the following circumstances:
1. When the satellite orbits pass through radiation belts such as the van-Allen belts around the Earth that could destroy the solar cells
2. Operations on the Moon or Mars where long periods of darkness require heavy batteries to supply power when solar cells would not have access to sunlight
3. Space missions in opaque atmospheres like Jupiter, where solar cells would be useless because of a lack of light.
4. At a distance far from the sun for long-duration missions where fuel cells, batteries and solar arrays would be too large and heavy.
5. Heating the electronics and storage batteries in the deep cold space at minus 245° F is necessary.
MEDICAL APPLICATION
The medical field finds a lot of applications with nuclear batteries due to their increased longevity and better reliability. It would be suited for medical devices like pacemakers, implanted deep fibrillation or other implanted devices that would otherwise require surgery to replace or repair the best box used in ‘cardiac pacemakers’. Batteries used in implantable cardiac pacemakers present unique challenges to their developers and manufacturers in terms of high levels of safety and reliability and it often poses threat to the end customer. In addition, the batteries must have longevity to avoid frequent replacement. The technological advances in leads/electrodes have reduced energy requirements by two orders of magnitude. Microelectronics advances sharply reduce internal current drain, concurrently decreasing size and increasing functionality, reliability and longevity. It is reported that about 600,000 pacemakers are implanted each year worldwide and the total number of people with various types of implanted pacemakers has already crossed 3,000,000. A cardiac pacemaker uses half of its battery power for cardiac stimulation and the other half for housekeeping tasks such as monitoring and data logging. The first implanted cardiac pacemaker used a nickel-cadmium rechargeable battery, later on, a zinc-mercury battery was developed and used which lasted for over two years. Lithium iodide battery, formed in 1972 made a real impact on implantable cardiac pacemakers and is on the way. But it draws a severe threat that lasts for about ten years and this is a serious problem. The lifetime solution is the nuclear battery.
MOBILE DEVICES
Xcell-N is a nuclear-powered laptop battery that can provide between seven and eight thousand times the life of a normal laptop battery-that is more than five years' worth of continuous power.
Nuclear batteries are about forgetting things around the usual charging, battery replacing and such bottlenecks. Since chemical batteries are just near the end of their life, we can’t expect much more from them, in its lowest accounts, a nuclear battery can endure at least up to five years. The Xcell-N is in continuous working for the last eight months and has not been turned off and has never been plugged into electrical power since. Nuclear batteries are going to replace conventional batteries and adaptors, so the future will be an exciting innovative new approach to powering portable devices.
AUTOMOBILES
Although it is in the initial stages of development, it is highly promised that nuclear batteries will find a sure niche in automobiles replacing the weary conventional iconic fuels there will be no case such as running out of fuel and running short of time. ‘Fox valley auto association, USA’ already conducted many seminars on the scopes and they are on the way to implementing this. Although the risks associated with the usage of nuclear batteries, even those concerned with legal restrictions are many, its advantages over the usual gasoline fuels are overcoming all the obstacles.
MILITARY APPLICATIONS
The army is undertaking a transformation into a more responsive, deployable, and sustainable force while maintaining high levels of lethality, survivability and versatility.
In unveiling this strategy, the final resource that fits quite beneficial is ‘the nuclear battery.
“TRACE photonics, U.S. Army Armaments Research, Development and Engineering Centre” has harnessed radioisotope power sources to provide very high energy density battery power to the men in action. Nuclear batteries are much lighter than chemical batteries and will last years, even decades. No power cords or transformers will be needed for the next generation of microelectronics in which voltage-matched supplies are built into components. A safe, long-life, reliable and stable temperature is available from the direct conversion of radioactive decay energy to electricity. This distributed energy source is well suited to active radio frequency equipment tags, sensors and ultra wide-band communication chips used on the modern battlefield.
CONCLUSION
The world of tomorrow that science fiction dreams of and technology manifests might be a very small one. It would reason that small devices would need small batteries to power them. The use of power as heat and electricity from radioisotopes will continue to be indispensable. As technology grows, the need for more power and more heat will undoubtedly grow along with it.
Clearly, the current research on nuclear batteries shows promise in future applications for sure. With the implementation of this new technology credibility and feasibility of the device will be heightened. The principal concern of nuclear batteries comes from the fact that it involves the use of radioactive materials. This means throughout the process of making a nuclear battery to final disposal, all radiation protection standards must be met. The economic feasibility of nuclear batteries will be determined by their applications and advantages. With several features being added to this little wonder and other parallel laboratory works going on, nuclear cells are going to be the next best thing ever invented in human history.